Abstract
Introduction
Hereditary alpha-Tryptasemia (HαT) is a group of genetically defined traits that share increased copy number of TPSAB1 gene encoding for both the α- and β-alleles (Lyons et al 2018). Increased copy number (CN) of the α-tryptase coding sequence in TPSAB1 on one or both alleles represents the genetic base of HαT (Lyons et al 2016). HαT is characterized by mild elevation in serum tryptase levels and a variety of mast cell (MC) activation symptoms, including recurrent anaphylaxis. Prevalence in the general population is up to 5%, that increases up to 20% in systemic mastocytosis (SM); it has been suggested that HαT might be a germline variant predisposing to SM development. In SM, HαT correlated with higher incidence of mediator-related symptoms (Greiner G et al, Blood 2021). Due to its complexity, the assay for TPSAB1 CN is performed in few centers and the actual prevalence of HαT in selected subsets is still to be elucidated. To this end, we screened for HαT 2 groups of subjects, the first with MC activation symptoms and no evidence of SM, the second with diagnosis of SM according to WHO-2016.
Methods
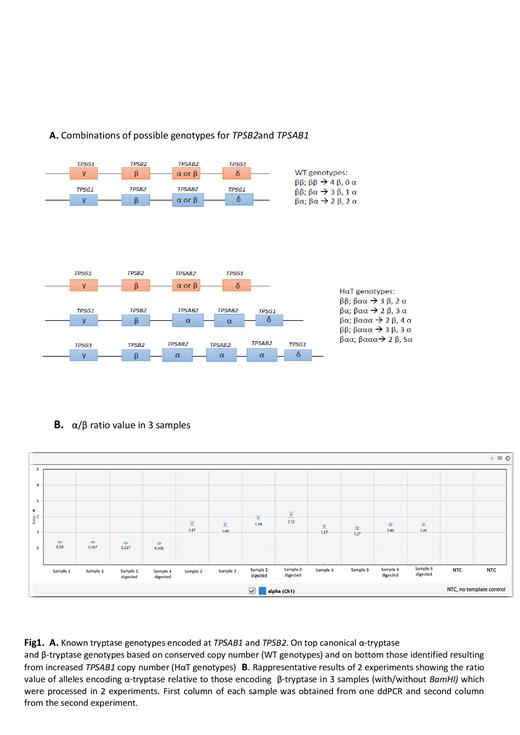
Droplet digital PCR (ddPCR) was used to measure CN variation (CNV) in TPSAB1 by adapting standard CNV ddPCR protocol to genotype for both TPSAB1 and TPSB2 (Fig.1A). The high homology between α and β encoding isoforms and the presence of paralogous genes in a single locus makes TPSAB1 CNV detection very challenging. ddPCR was performed on genomic DNA with/without BamHI, using the PrimePCR ddPCR Copy Number reference AP3B1 (BioRad). Accuracy and precision of the ddPCR protocol was assessed by analyzing 10 samples in triplicate in 3 separate experiments. Data robustness and repeatability can be appreciated in Fig 1B.
Results
We studied 41 subjects with mediator-related symptoms and augmented basal serum tryptase (BST) (cohort 1) and 150 patients with ascertained diagnosis of mastocytosis (cohort 2). The BST threshold established for cohort 1 was equal or higher than 11 mcg/L. Median age was 64.7 yr, males 54%; median BST levels was 15.3 (range 12.3-21 mcg/L); 29% of the pts had history of anaphylaxis.
In cohort 2, 134/150 (89.3%) pts had a diagnosis of SM, whereas 13/150 (8,6%) were Cutaneous Mastocytosis (CM). Among SM patients, 113(84.3%) presented with non-advanced SM variants. Advanced forms including aggressive SM (ASM) and SM with an associated hematological neoplasm (SM-AHN) were diagnosed in 6 (4.5%) and 8 (6%) respectively. In 3 pts, SM subtype was not available. Median age was 49 yr, males 55%; 41.7% of the pts had history of anaphylaxis.
HαT was documented in 27 (65.9%) subjects in cohort 1, and 14 (9.3%) in cohort 2. In cohort 1, 3 α-tryptase (3α) copy number was observed more frequently (59.2% of HαT+ pts); conversely 3α and 2α-tryptase copy number were observed at a similar rate (42,8%) in cohort 2.
HαT+ pts in cohort 1 presented significantly higher BST (17.1 vs 12.05 mgc/L, P<0.001), as previously reported (Greiner G et al, Blood 2021); however, occurrence of mediator related symptoms was comparable to HαT wt, 72% vs 71.4% , respectively; likewise for anaphylaxis (28% in HαT+ vs 33%).
In cohort 2, BST levels were similar in HαT+ and HαT wt pts (24.6 and 24.3 mcg/L), as were anaphylaxis episodes (50% and 41%, respectively). A trend for lower MC burden in HαT+ as assessed by flow cytometry was demonstrated (% of bone marrow MC: 0.01% in HαT+ vs 0.07%) whereas no meaningful differences emerged regarding the symptom burden. In addition, a lower prevalence of KIT 816V mutation was observed in HαT+ (71.4% vs 89.5%; p=0.073).
Conclusions
In our study HαT+ was observed in around 10% of patients with SM, a prevalence lower than previously reported (Greiner et al, Blood 2021) and remarkably lower than in a selected cohort of subjects with raised BTL and history of mediator-released symptoms (66%). ddPCR represents a suitable method to investigate the presence of CNV in the α-tryptase coding sequence. Genetic testing for HαT+ should be considered in the diagnostic workout of patients presenting with anaphylaxis or MC mediator-related symptoms and no suspicion/evidence of SM. The clinical correlates of HαT in SM remain to be fully ascertained.
Supported by IMH no.GR-2016-02362631 and AIRC, Mynerva project no21267
Elena: CELGENE: Other: funding for meeting participation; PFIZER: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees. Vannucchi: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal